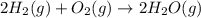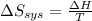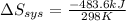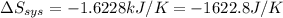Consider the following reaction at 298 k:
2h2(g) + o2(g) --> 2h2o(g) deltah= -483.6...

Chemistry, 07.12.2019 02:31, helloitschump0vfdz
Consider the following reaction at 298 k:
2h2(g) + o2(g) --> 2h2o(g) deltah= -483.6 kj
calculate the following quantities.
delta s sys=
delta s surr=
delta s univ=

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 00:00, maronetham6253
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
Do you know the correct answer?
Questions in other subjects:


English, 20.05.2021 14:00

History, 20.05.2021 14:00


Mathematics, 20.05.2021 14:00

Computers and Technology, 20.05.2021 14:00

English, 20.05.2021 14:00

Chemistry, 20.05.2021 14:00

Chemistry, 20.05.2021 14:00

Mathematics, 20.05.2021 14:00


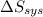 = -1622.8 J/K
= -1622.8 J/K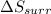 = -94.6 J/K
= -94.6 J/K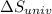 = 0 J/K
= 0 J/K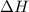 = -483.6 kJ
= -483.6 kJ