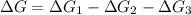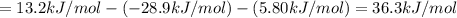Consider these hypothetical chemicalreactions:
{\rm a \rightleftharpoons b}, \quad\delta g =...

Chemistry, 07.12.2019 02:31, queenkimm26
Consider these hypothetical chemicalreactions:
{\rm a \rightleftharpoons b}, \quad\delta g = 13.2 kj/mol
{\rm b \rightleftharpoons c}, \quad\delta g = -28.9 kj/mol
{\rm c \rightleftharpoons d},\quad \delta g = 5.80 kj/mol
what is the free energy, delta g, for the overall reaction, \rm a \rightleftharpoons d ?
express your answer numerically inkilojoules per mole.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, eweqwoewoji
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1


Do you know the correct answer?
Questions in other subjects:





Computers and Technology, 21.07.2021 01:50


Mathematics, 21.07.2021 01:50


Mathematics, 21.07.2021 01:50

Mathematics, 21.07.2021 01:50


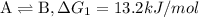 ...[1]
...[1]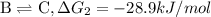 ...[2]
...[2]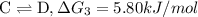 ...[3]
...[3]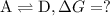 ...[4]
...[4]