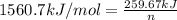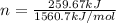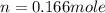Chemistry, 06.12.2019 23:31, Justus4215
The combustion of how many moles of ethane (c2h6) would be required to heat 851 g of water from 25.0°c to 98.0°c? (assume liquid water is formed during the combustion.)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 07:00, ceeejay0621
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 18:00, kamjay2006
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
Do you know the correct answer?
The combustion of how many moles of ethane (c2h6) would be required to heat 851 g of water from 25.0...
Questions in other subjects:


Chemistry, 21.10.2020 14:01


History, 21.10.2020 14:01


Mathematics, 21.10.2020 14:01


Biology, 21.10.2020 14:01

Chemistry, 21.10.2020 14:01


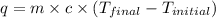
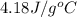
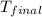 = final temperature =
= final temperature = 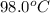
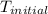 = initial temperature =
= initial temperature = 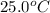
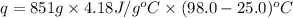
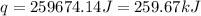 (1 kJ = 1000 J)
(1 kJ = 1000 J)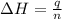
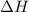 = enthalpy of combustion of ethane = 1560.7 kJ/mol (standard value)
= enthalpy of combustion of ethane = 1560.7 kJ/mol (standard value)