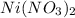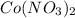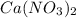Chemistry, 06.12.2019 21:31, blueval3tine
Astudent was given a solid containing a mixture of nitrate salts. the sample completely dissolved in water, and upon addition of dilute hcl, no precipitate formed. the ph was lowered to about 1 and h2s was bubbled through the solution. no precipitate formed. the ph was adjusted to 8 and h2s was again bubbled in. this time, a precipitate formed. which compounds might have been present in the unknown?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, lukeakalucas
Alarge marble is dropped in a graduated cylinder with 35ml of water in it. the water level increases to 49ml. what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
Do you know the correct answer?
Astudent was given a solid containing a mixture of nitrate salts. the sample completely dissolved in...
Questions in other subjects:







History, 25.09.2019 20:30

Biology, 25.09.2019 20:30

Mathematics, 25.09.2019 20:30

English, 25.09.2019 20:30