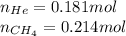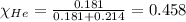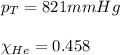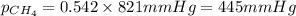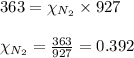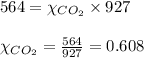Amixture of helium and methane gases, at a total pressure of 821 mm hg, contains 0.723 grams of helium and 3.43 grams of methane. what is the partial pressure of each gas in the mixture?
phe = mm hg
pch4 = mm hg
2.) a mixture of nitrogen and carbon dioxide gases contains nitrogen at a partial pressure of 363 mm hg and carbon dioxide at a partial pressure of 564 mm hg. what is the mole fraction of each gas in the mixture?
xn2 =
xco2 =

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 23.06.2019 00:00, kittenalexis68
How many atoms or molecules are there in a mole of a substance?
Answers: 1
Do you know the correct answer?
Amixture of helium and methane gases, at a total pressure of 821 mm hg, contains 0.723 grams of heli...
Questions in other subjects:

German, 26.01.2021 01:00




Mathematics, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00



Mathematics, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00


 .....(1)
.....(1)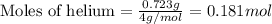
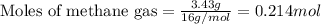
 .......(2)
.......(2) ......(3)
......(3)