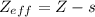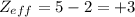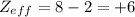Chemistry, 06.12.2019 20:31, jadepotts3965
The shielding of electrons gives rise to an effective nuclear charge,  , which explains why boron is larger than oxygen. estimate the approximate
, which explains why boron is larger than oxygen. estimate the approximate  felt by a valence electron of boron and oxygen, respectively? a. +5 and +8b. +3 and +6c. +5 and +6d. +3 and +8e. +1 and +4
felt by a valence electron of boron and oxygen, respectively? a. +5 and +8b. +3 and +6c. +5 and +6d. +3 and +8e. +1 and +4

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, notkeandre9
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1


Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
Do you know the correct answer?
The shielding of electrons gives rise to an effective nuclear charge, [tex]z_{eff}[/tex], which expl...
Questions in other subjects:



Mathematics, 26.10.2020 05:00


Mathematics, 26.10.2020 05:00

Arts, 26.10.2020 05:00


English, 26.10.2020 05:00


Mathematics, 26.10.2020 05:00


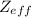 of the valence electron of boron and oxygen, we need to use the next equation:
of the valence electron of boron and oxygen, we need to use the next equation: