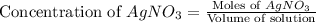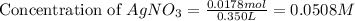Chemistry, 06.12.2019 19:31, orlando19882000
Suppose a 2.95 g of potassium iodide is dissolved in 350. ml of a 62.0 m m aqueous solution of silver nitrate. calculate the final molarity of iodide anion in the solution. you can assume the volume of the solution doesn't change when the potassium iodide is dissolved in it. be sure your answer has the correct number of significant digits

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1



Chemistry, 23.06.2019 15:30, 101EXPERIENCE
Which term defines a type of oxygen that forms a protective layer miles above the earth a. fossil fuel b. smog c. pollution d. ozone
Answers: 2
Do you know the correct answer?
Suppose a 2.95 g of potassium iodide is dissolved in 350. ml of a 62.0 m m aqueous solution of silve...
Questions in other subjects:

English, 28.05.2021 01:00




English, 28.05.2021 01:00



Mathematics, 28.05.2021 01:00


Computers and Technology, 28.05.2021 01:00


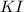 and
and 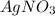 .
.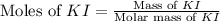
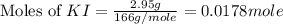
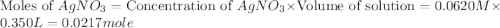
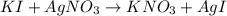
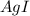
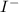 anion = Moles of
anion = Moles of 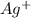 cation = 0.0178 moles
cation = 0.0178 moles