
Give the chemical symbol for the element with the ground‑state electron configuration [ ar ] 4 s 2 3 d 1 . symbol: determine the quantum numbers n and ℓ and select all possible values for m ℓ for each subshell of the element. 4 s n = 4 s ℓ = the possible values of m ℓ for the 4 s subshell are 0 − 1 , 0 , + 1 − 2 , − 1 , 0 , + 1 , + 2 − 3 , − 2 , − 1 , 0 , + 1 , + 2 , + 3 3 d n = 3 d ℓ = the possible values of m ℓ for the 3 d subshell are − 1 , 0 , + 1 − 2 , − 1 , 0 , + 1 , + 2 − 3 , − 2 , − 1 , 0 , + 1 , + 2 , + 3 0

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3


Chemistry, 23.06.2019 05:30, choatefarmsus
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1

Chemistry, 23.06.2019 07:00, jaydenboi604
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
Do you know the correct answer?
Give the chemical symbol for the element with the ground‑state electron configuration [ ar ] 4 s 2 3...
Questions in other subjects:


Spanish, 26.06.2019 15:20





Social Studies, 26.06.2019 15:20


Biology, 26.06.2019 15:20

Mathematics, 26.06.2019 15:20


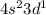 .
.



