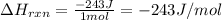4). one mole of monoclinic sulfur at 25c was placed in a constant-pressure calorimeter whose heat capacity (c) was 1620 j/k. the temperature of the calorimeter increased by 0.150 co when the sulfur changed from the monoclinic to the orthorhombic form. calculate the enthalpy change for the process s(monoclinic) s(orthorhombic).

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
Do you know the correct answer?
4). one mole of monoclinic sulfur at 25c was placed in a constant-pressure calorimeter whose heat c...
Questions in other subjects:

Computers and Technology, 06.03.2021 03:20





English, 06.03.2021 03:20


Mathematics, 06.03.2021 03:20

Mathematics, 06.03.2021 03:20


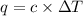
 = change in temperature =
= change in temperature = 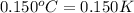 (Change remains same)
(Change remains same)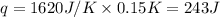
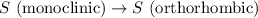
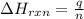
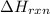 = enthalpy change of the reaction
= enthalpy change of the reaction