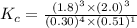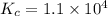Chemistry, 06.12.2019 06:31, btcastongia
Part a what is the numerical value of kc for the following reaction if the equilibrium mixture contains 0.51 m c3h6o, 0.30 m o2, 1.8 m co2, and 2.0 m h2o? c3h6o(g)+4o2(g)⇌3co2(g)+3h2o(g)
a) 2.4 × 101b) 1.1 × 104c) 8.9 × 10-5d) 4.3 × 10-2

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1


Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
Do you know the correct answer?
Part a what is the numerical value of kc for the following reaction if the equilibrium mixture conta...
Questions in other subjects:


Chemistry, 21.09.2020 14:01



History, 21.09.2020 14:01


Mathematics, 21.09.2020 14:01





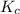 .
.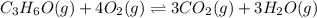
![K_c=\frac{[CO_2]^3\times [H_2O]^3}{[O_2]^4\times [C_3H_6O]^1}](/tpl/images/0406/0681/8cf44.png)