

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, psychocatgirl1
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 23.06.2019 01:00, zitterkoph
Which of the following is a physical change? a. burning a piece of wood b. sawing a piece of wood in half c. rust forming on an iron fence d. a copper roof changing color from orange to green
Answers: 1
Do you know the correct answer?
When nh3(g) reacts with o2(g), the products of the combustion are no(g) and h2o(g). what volume of o...
Questions in other subjects:


Biology, 11.10.2020 01:01

English, 11.10.2020 01:01

Arts, 11.10.2020 01:01




History, 11.10.2020 01:01


SAT, 11.10.2020 01:01


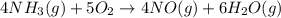
 of oxygen gas
of oxygen gas



