Chemistry, 06.12.2019 05:31, AaronMicrosoft15
If the equilibrium constant for a two-electron redox reaction at 298 k is 1.0×10−4, calculate the corresponding δg∘ and e∘cel under standard conditions.
δg∘ =
e∘cell =

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, applereams
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2
Do you know the correct answer?
If the equilibrium constant for a two-electron redox reaction at 298 k is 1.0×10−4, calculate the co...
Questions in other subjects:

Biology, 04.08.2019 21:30

Social Studies, 04.08.2019 21:30



History, 04.08.2019 21:30

Mathematics, 04.08.2019 21:30

Social Studies, 04.08.2019 21:30


Computers and Technology, 04.08.2019 21:30


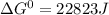
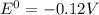
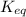
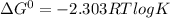
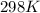
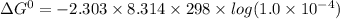
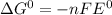
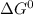 = gibbs free energy = 22823J
= gibbs free energy = 22823J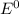 = standard emf
= standard emf