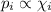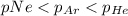Chemistry, 06.12.2019 05:31, zachcochran2007
Amixture of he, ne, and ar gases at a particular temperature has partial pressures of 0.75 atm he, 0.30 atm ne, and 0.62 atm ar. which of the following shows the correct trend for the mole fractions of the component gases?
a. latex: \chi_{ne}< \chi_{ar}< \chi_{he}b. latex: \chi_{ar}< \chi_{ne}< \chi_{he}c. latex: \chi_{he}< \chi_{ar}< \chi_{ne}d. latex: \chi_{he}< \chi_{ne}< \chi_{ar}

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:30, TheViperMlg23676
What causes most sediment to wash or fall into a river
Answers: 1

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
Do you know the correct answer?
Amixture of he, ne, and ar gases at a particular temperature has partial pressures of 0.75 atm he, 0...
Questions in other subjects:


History, 30.06.2020 18:01

Mathematics, 30.06.2020 18:01

Mathematics, 30.06.2020 18:01


Chemistry, 30.06.2020 18:01


Mathematics, 30.06.2020 18:01

Mathematics, 30.06.2020 18:01

Mathematics, 30.06.2020 18:01


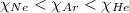
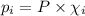
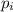 = partial pressure of the i component
= partial pressure of the i component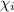 = Mole fraction of the i component
= Mole fraction of the i component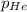 = 0.75 atm
= 0.75 atm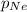 = 0.30 atm
= 0.30 atm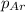 = 0.62 atm
= 0.62 atm