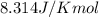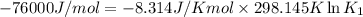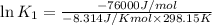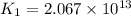Chemistry, 06.12.2019 04:31, emilybomar7466
Consider the reaction 4 hcl(g) + o2(g) =2 h2o(g) + 2 cl2(g) using the standard thermodynamic data in the tables linked above, calculate the equilibrium constant for this reaction at 298.15k.
delta g (kj/mol)
hcl=-95.3
o2=0
h2o=-228.6
cl2=0

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 04:30, akeemedwards12
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2
Do you know the correct answer?
Consider the reaction 4 hcl(g) + o2(g) =2 h2o(g) + 2 cl2(g) using the standard thermodynamic data in...
Questions in other subjects:

Mathematics, 30.05.2021 22:20



Mathematics, 30.05.2021 22:20



Mathematics, 30.05.2021 22:20



English, 30.05.2021 22:20


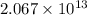 .
.![\Delta G^o_{rxn}=\sum [n\times \Delta G^o_f(product)]-\sum [n\times \Delta G^o_f(reactant)]](/tpl/images/0405/8552/b00b4.png)
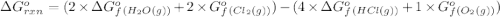
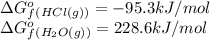
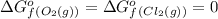 (pure element)
(pure element)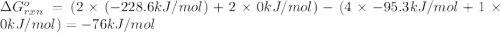
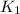 (at 25°C) for given value of Gibbs free energy, we use the relation:
(at 25°C) for given value of Gibbs free energy, we use the relation: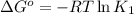
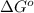 = Gibbs free energy = -76 kJ/mol = -76000 J/mol
= Gibbs free energy = -76 kJ/mol = -76000 J/mol