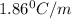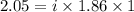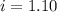Chemistry, 06.12.2019 03:31, Unkn0wn3815
You are given a 1.00 molal solution of an unknown solute dissolved in water and told that you must determine if the solute is a strong electrolyte, a weak electrolyte, or a nonelectrolyte. on testing, you find that the freezing point of the solution is -2.05 degrees celsius. is this enough information to answer the question? the molal freezing point depression constant for water is 1.86 °c/m. o no, this is not enough information o yes, the solute is a nonelectrolyte yes, the solute is a weak electrolyte yes, the solute is a strong electrolyte

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, officialalex6330
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 23.06.2019 00:30, hdhshshs741
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 02:30, hailee232
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
Do you know the correct answer?
You are given a 1.00 molal solution of an unknown solute dissolved in water and told that you must d...
Questions in other subjects:


Biology, 16.04.2021 01:10


Mathematics, 16.04.2021 01:10




Mathematics, 16.04.2021 01:10

Mathematics, 16.04.2021 01:10

Chemistry, 16.04.2021 01:10


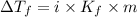
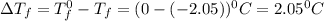 = Depression in freezing point
= Depression in freezing point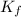 = freezing point constant =
= freezing point constant =