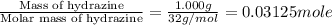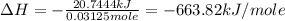Chemistry, 06.12.2019 03:31, isabeltorres5
A1.000 gram sample of the rocket fuel hydrazine (n2h4) is burned in a bomb calorimeter. the temperature rises from 24.62°c to 28.16°c. the heat capacity of the calorimeter (including the water) is 5860 j/°c. calculate the molar heat of combustion of hydrazine, in kj/mole.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, peaceouthjkdrb2398
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 22:00, cooljariel11
Give more examples of this type of heat transfer:
Answers: 1

Chemistry, 23.06.2019 02:20, alejandraluna95
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
Do you know the correct answer?
A1.000 gram sample of the rocket fuel hydrazine (n2h4) is burned in a bomb calorimeter. the temperat...
Questions in other subjects:

Mathematics, 28.08.2021 16:10



Chemistry, 28.08.2021 16:20

English, 28.08.2021 16:20


Physics, 28.08.2021 16:20

Law, 28.08.2021 16:20

Social Studies, 28.08.2021 16:30


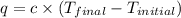
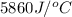
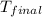 = final temperature =
= final temperature = 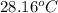
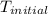 = initial temperature =
= initial temperature = 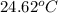
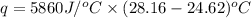
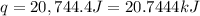
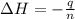
 = enthalpy change = ?
= enthalpy change = ?