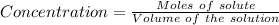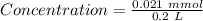Chemistry, 06.12.2019 02:31, bvaughn4152
Achemist prepares a solution of nickel(ii) chloride nicl2 by measuring out 21.μmol of nickel(ii) chloride into a 200.ml volumetric flask and filling the flask to the mark with water. calculate the concentration in /mmoll of the chemist's nickel(ii) chloride solution. round your answer to 2 significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1


Do you know the correct answer?
Achemist prepares a solution of nickel(ii) chloride nicl2 by measuring out 21.μmol of nickel(ii) chl...
Questions in other subjects:



Business, 01.02.2021 20:40



Mathematics, 01.02.2021 20:40

History, 01.02.2021 20:40

Mathematics, 01.02.2021 20:40


History, 01.02.2021 20:40


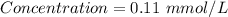
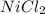 = 21 μmol
= 21 μmol