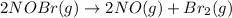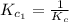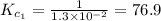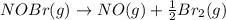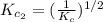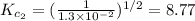Chemistry, 06.12.2019 02:31, taylorb9893
The equilibrium constant for the reaction 2no(g)+br2(g)⥫⥬==2nobr(g) is kc=1.3×10−2 at 1000 k. at this temperature does the equilibrium favor no and br2, or does it favor nobr? calculate kc for 2nobr(g)⥫⥬==2no(g)+br2(g). calculate kc for nobr(g)⥫⥬==no(g)+12br2(g).

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1


Chemistry, 23.06.2019 02:30, paulinahunl17
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
Do you know the correct answer?
The equilibrium constant for the reaction 2no(g)+br2(g)⥫⥬==2nobr(g) is kc=1.3×10−2 at 1000 k. at thi...
Questions in other subjects:


English, 08.07.2019 18:50

Physics, 08.07.2019 18:50


SAT, 08.07.2019 18:50

History, 08.07.2019 18:50





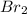 .
.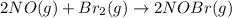
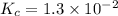 .
.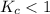 that means equilibrium lies to the left side. Thus, the equilibrium favors NO and
that means equilibrium lies to the left side. Thus, the equilibrium favors NO and