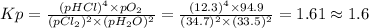Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2(g) + 2h2o(g) → 4hcl(g) + o2(g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound pressure at equilibrium cl2 34.7atm h2o 33.5atm hcl 12.3atm o2 94.9atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2
Do you know the correct answer?
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2(g) + 2h2o(g) → 4hcl(...
Questions in other subjects:



Mathematics, 05.07.2019 02:00

Mathematics, 05.07.2019 02:00





History, 05.07.2019 02:00

Mathematics, 05.07.2019 02:00