

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 18:40, bananaslada
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
Do you know the correct answer?
16.34 g of cuso4 dissolved in water giving out 55.51 kj and 25.17 g cuso4•5h2o absorbs 95.31 kj. fro...
Questions in other subjects:


Chemistry, 05.12.2020 01:00

Chemistry, 05.12.2020 01:00


History, 05.12.2020 01:00




Mathematics, 05.12.2020 01:00

Chemistry, 05.12.2020 01:00


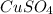 dissolved
dissolved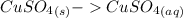 (Eq. 1)
(Eq. 1)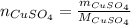
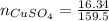
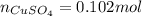
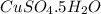 dissolved
dissolved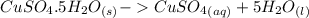 (Eq. 2)
(Eq. 2)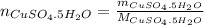
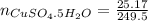
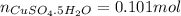
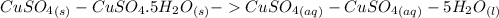 ΔH = -541.85-944.77
ΔH = -541.85-944.77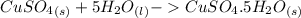 ΔH = -1486.62 kJ/mol
ΔH = -1486.62 kJ/mol




