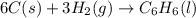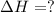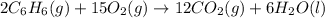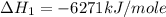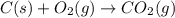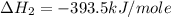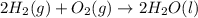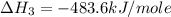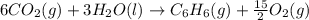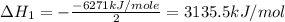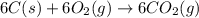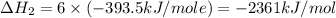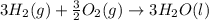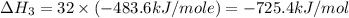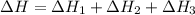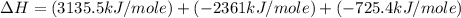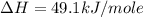Chemistry, 05.12.2019 23:31, carlinryan
Using the equations 2 c₆h₆ (l) + 15 o₂ (g) → 12 co₂ (g) + 6 h₂o (g)∆h° = -6271 kj/mol c (s) + o₂ (g) → co₂ (g) ∆h° = -393.5 kj/mol 2 h₂ (g) + o₂ (g) → 2 h₂o (g) ∆h° = -483.6 kj/mol determine the enthalpy for the reaction 6 c (s) + 3 h₂ (g) → c₆h₆ (l).

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Aidanjsauer
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2


Chemistry, 23.06.2019 03:30, nikkio4
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus. b) the number of neutrons it contains in its nucleus. c) the number of protons it has in a cloud around the nucleus. d) the number of neutrons it has in a cloud around the nucleus. e) the number of electrons it exchanges with its neighbors.
Answers: 1
Do you know the correct answer?
Using the equations 2 c₆h₆ (l) + 15 o₂ (g) → 12 co₂ (g) + 6 h₂o (g)∆h° = -6271 kj/mol c (s) + o₂ (g)...
Questions in other subjects:

English, 02.09.2019 15:50

History, 02.09.2019 15:50


Mathematics, 02.09.2019 15:50

History, 02.09.2019 15:50

Mathematics, 02.09.2019 15:50



History, 02.09.2019 15:50

Social Studies, 02.09.2019 15:50


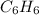 will be,
will be,