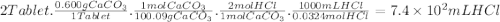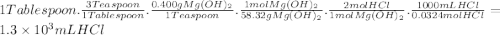The daily output of stomach acid (gastric juice) is 1000 to 2000 ml. prior to a meal, stomach acid (hcl) typically has a ph of 1.49.b.) one chewable tablet of the antacid maalox contains 600. mg of caco3. enter the neutralization equation. express your answer as a molecular equation including phasesc.) given that one chewable tablet of the antacid maalox contains 600. mg of caco3, calculate the milliliters of stomach acid neutralized by two tablets of maalox. express the volume in milliliters to two significant figures. d.) the antacid milk of magnesia contains 400. mg of mg(oh)2 per teaspoon. enter the neutralization equation. express your answer as a molecular equation including phases. e.) given that the antacid milk of magnesia contains 400. mg of mg(oh)2 per teaspoon, calculate the number of milliliters of stomach acid that are neutralized by 1 tablespoon of milk of magnesia. (1 tablespoon = 3 teaspoons.)express the volume in milliliters to two significant figures.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, 21brooklynmartin
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2


Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 20:30, Schoolworkspace453
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
Do you know the correct answer?
The daily output of stomach acid (gastric juice) is 1000 to 2000 ml. prior to a meal, stomach acid (...
Questions in other subjects:

Mathematics, 30.07.2020 20:01



Mathematics, 30.07.2020 20:01


Mathematics, 30.07.2020 20:01

Spanish, 30.07.2020 20:01



Mathematics, 30.07.2020 20:01