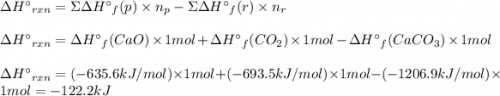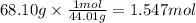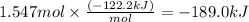Chemistry, 05.12.2019 22:31, 20stirltrer
At 850°c, caco3 undergoes substantial decomposition to yield cao and co2. assuming that the δh o f values of the reactant and products are the same at 850°c as they are at 25°c, calculate the enthalpy change (in kj) if 68.10 g of co2 is produced in one reaction.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, ashtonbillups
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3


Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
Do you know the correct answer?
At 850°c, caco3 undergoes substantial decomposition to yield cao and co2. assuming that the δh o f v...
Questions in other subjects:

Mathematics, 20.05.2021 22:10

Social Studies, 20.05.2021 22:10



English, 20.05.2021 22:10

Social Studies, 20.05.2021 22:10




Mathematics, 20.05.2021 22:10