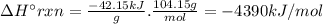Chemistry, 05.12.2019 20:31, leeamation31
Styrene, c8h8, is one of the substances used in the production of synthetic rubber. when styrene burns in oxygen to form carbon dioxide and liquid water under standard-state conditions at 25°c, 42.15 kj are released per gram of styrene. find the standard enthalpy of formation of styrene at 25°c.
(given: ? h°f[co2(g)] = –393.5 kj/mol, ? h°f[h2o(l)] = –285.8 kj/mol, ? h°f[h2o(g)] = –241.8 kj/mol)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1
Do you know the correct answer?
Styrene, c8h8, is one of the substances used in the production of synthetic rubber. when styrene bur...
Questions in other subjects:

Mathematics, 21.04.2021 21:10

English, 21.04.2021 21:10

Mathematics, 21.04.2021 21:10

Mathematics, 21.04.2021 21:10





Mathematics, 21.04.2021 21:10