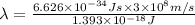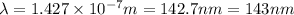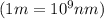Chemistry, 05.12.2019 20:31, arianawelsh123l
It takes 839./kjmol to break a carbon-carbon triple bond. calculate the maximum wavelength of light for which a carbon-carbon triple bond could be broken by absorbing a single photon.
round your answer to 3 significant digits in nm.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, girlchamp654
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 21.06.2019 21:10, danielahchf
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:30, azzyla2003
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
Do you know the correct answer?
It takes 839./kjmol to break a carbon-carbon triple bond. calculate the maximum wavelength of light...
Questions in other subjects:




Mathematics, 04.05.2021 05:10


Geography, 04.05.2021 05:10

Mathematics, 04.05.2021 05:10

English, 04.05.2021 05:10



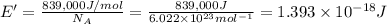
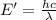 (Using planks equation)
(Using planks equation)