
To identify a diatomic gas (x2), a researcher carried out the following experiment: she weighed an empty 5.8-l bulb, then filled it with the gas at 1.00 atm and 21.0 ∘c and weighed it again. the difference in mass was 6.7 g. identify the gas. express your answer as a chemical formula.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3


Chemistry, 23.06.2019 07:30, danielahumajova6
How do you interpret a chromagram for what mixtures contain?
Answers: 1
Do you know the correct answer?
To identify a diatomic gas (x2), a researcher carried out the following experiment: she weighed an...
Questions in other subjects:



Mathematics, 15.07.2019 22:30

History, 15.07.2019 22:30



English, 15.07.2019 22:30




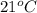 = (21 + 273) K = 294 K, mass = 6.7 g
= (21 + 273) K = 294 K, mass = 6.7 g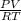
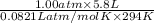
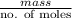
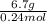
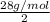
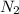 .
.



