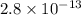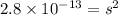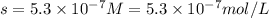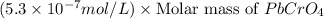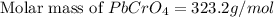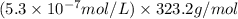Chemistry, 05.12.2019 18:31, BrainlyAvenger
G[mcquarrie 22-7] the value of ksp for pbcro4(s) in equilibrium with water at 25◦c is 2.8 · 10−13m2 . write the chemical equation that represents the solubility equilibrium for pbcro4(s) and calculate its solubility in grams per liter in water at 25◦c.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 10:30, mikemofun9079
Silver is a white metal that is an excellent conductor. silver tarnishes when exposed to air and light. the density of silver is 10.49 g/cm3. the melting point is 962oc and the boiling point is 2000oc. a chemical property of silver is
Answers: 3
Do you know the correct answer?
G[mcquarrie 22-7] the value of ksp for pbcro4(s) in equilibrium with water at 25◦c is 2.8 · 10−13m2...
Questions in other subjects:


Mathematics, 24.04.2021 03:40


Mathematics, 24.04.2021 03:40



Mathematics, 24.04.2021 03:40




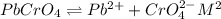
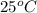 is,
is, 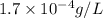
![K_{sp}=[Pb^{2+}][CrO_4^{2-}]](/tpl/images/0404/8064/04f28.png)
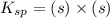
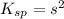
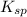 =
=