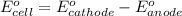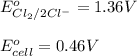Chemistry, 05.12.2019 18:31, eyeneedalife
The overall reaction 2co3+(aq) + 2cl–(aq) → 2co2+(aq) + cl2(g) has the standard cell voltage e°cell = 0.46 v. given e° = 1.36 v for the reaction cl2(g) + 2e– → 2cl–(aq), calculate the standard reduction potential for the following the half reaction at 25°c: co3+ + e– → co2+

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3


Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 17:00, BREBRE8932
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
Do you know the correct answer?
The overall reaction 2co3+(aq) + 2cl–(aq) → 2co2+(aq) + cl2(g) has the standard cell voltage e°cell...
Questions in other subjects:

Chemistry, 23.10.2021 01:00

English, 23.10.2021 01:00

Mathematics, 23.10.2021 01:00

Social Studies, 23.10.2021 01:00

History, 23.10.2021 01:00

Mathematics, 23.10.2021 01:00

Mathematics, 23.10.2021 01:00


Mathematics, 23.10.2021 01:00

Chemistry, 23.10.2021 01:00



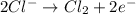
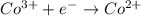
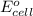 of the reaction, we use the equation:
of the reaction, we use the equation: