
Chemistry, 05.12.2019 05:31, sumayyahjj
How much heat (in kj) is required to warm 13.0 g of ice, initially at -10.0 ∘c, to steam at 111.0 ∘c? the heat capacity of ice is 2.09 j/g⋅∘c and that of steam is 2.01 j/g⋅∘c, the heat of fusion for water is 6.02 kj/mol, and the heat of vaporization for water is 40.7 kj/mol.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1
Do you know the correct answer?
How much heat (in kj) is required to warm 13.0 g of ice, initially at -10.0 ∘c, to steam at 111.0 ∘c...
Questions in other subjects:




History, 14.03.2020 03:21



History, 14.03.2020 03:21

Mathematics, 14.03.2020 03:21



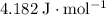 , the melting point of water is
, the melting point of water is 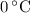 , and that the boiling point of water is
, and that the boiling point of water is 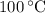 .
.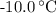 ice to steam at
ice to steam at 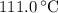 .
.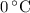 . The change in temperature would be
. The change in temperature would be 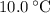 .Step two: supply the heat of fusion to convert that 13.0 gram of ice to water.Step three: heat the 13.0 gram of water from
.Step two: supply the heat of fusion to convert that 13.0 gram of ice to water.Step three: heat the 13.0 gram of water from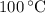 . The change in temperature would be
. The change in temperature would be 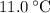 .Energy required for step one, three, and five
.Energy required for step one, three, and five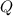 required to raise the temperature of an object by a
required to raise the temperature of an object by a  :
: 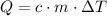 .
.  is the specific heat of this substance,
is the specific heat of this substance, 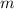 is the mass of the substance, and
is the mass of the substance, and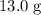 .
.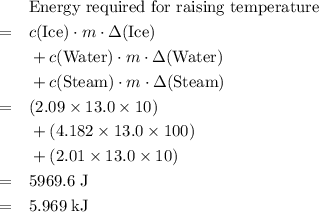 .
.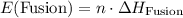 .
.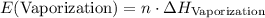 .
.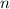 is the number of moles of the substance.
is the number of moles of the substance.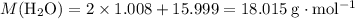 .
.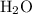 molecules in
molecules in 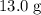 :
: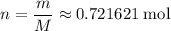 .
. 
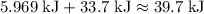 .
.




