
Chemistry, 05.12.2019 05:31, Damagingawsomeness2
A50.0 ml sample containing cd2+ and mn2+ was treated with 58.4 ml of 0.0400 m edta . titration of the excess unreacted edta required 15.9 ml of 0.0130 m ca2+ . the cd2+ was displaced from edta by the addition of an excess of cn− . titration of the newly freed edta required 22.4 ml of 0.0130 m ca2+ . what are the concentrations of cd2+ and mn2+ in the original solution?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3
Do you know the correct answer?
A50.0 ml sample containing cd2+ and mn2+ was treated with 58.4 ml of 0.0400 m edta . titration of th...
Questions in other subjects:

Mathematics, 24.04.2021 22:30

Mathematics, 24.04.2021 22:30

Mathematics, 24.04.2021 22:30

Mathematics, 24.04.2021 22:30




Mathematics, 24.04.2021 22:30



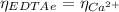
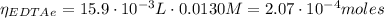
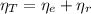 (1)
(1)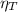 : is the total moles of EDTA,
: is the total moles of EDTA, 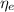 : is the EDTA excess moles and
: is the EDTA excess moles and 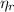 : is the EDTA moles that react with Cd²⁺ and Mn²⁺
: is the EDTA moles that react with Cd²⁺ and Mn²⁺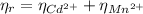 (2)
(2)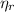 :
: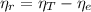
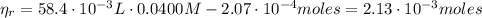
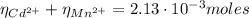 (3)
(3)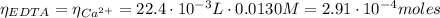
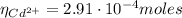
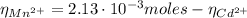
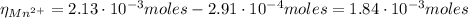
![[Cd^{2+}] = \frac{2.91 \cdot 10^{-4} moles}{50.0\cdot 10^{-3}L} = 5.82 \cdot 10^{-3} M](/tpl/images/0404/1338/54447.png)
![[Mn^{2+}] = \frac{1.84 \cdot 10^{-3} moles}{50.0\cdot 10^{-3}L} = 0.037M](/tpl/images/0404/1338/68079.png)




