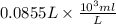Chemistry, 04.12.2019 21:31, magicalforlife
You have 0.500 l of an 0.250 m acetate buffer solution (i. e. [hc₂h₃o₂] + [c₂h₃o₂⁻] = 0.250 m) at ph 3.50. how many ml of 1.000 m naoh must you add in order to change the ph to 5.25? acetic acid has a pka of 4.74.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 23:30, emmalado45
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 05:00, MoneyMike42
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3

Chemistry, 23.06.2019 08:00, george27212
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
Do you know the correct answer?
You have 0.500 l of an 0.250 m acetate buffer solution (i. e. [hc₂h₃o₂] + [c₂h₃o₂⁻] = 0.250 m) at ph...
Questions in other subjects:




Mathematics, 05.11.2019 14:31


Mathematics, 05.11.2019 14:31



Physics, 05.11.2019 14:31


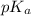 is as follows.
is as follows.![pK_{a} + log \frac{[CH_{3}COO^{-}]}{[CH_{3}COOH]}](/tpl/images/0403/2718/85431.png)
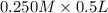
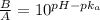
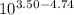
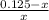 = 0.0575
= 0.0575![log \frac{[B]}{[A]}](/tpl/images/0403/2718/93cf8.png)
![log \frac{[B]}{[A]} = 10^{5.25 - 4.74}](/tpl/images/0403/2718/76315.png)
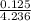
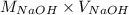 = (0.118 - 0.0295) moles
= (0.118 - 0.0295) moles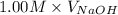 = 0.0885 moles
= 0.0885 moles