
Chemistry, 04.12.2019 06:31, jaedenevan062907
Which of the following statements explain why the van der waals equation must be used to describe real gases? x. interactions between gas molecules reduces the temperature of the gas in the sample y. the non-zero volumes of gas particles effectively decrease the amount of "empty space" between them z. the molecular attractions between particles of gas decreases the pressure exerted by the gas

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, Bryanguzman2004
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3



Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1
Do you know the correct answer?
Which of the following statements explain why the van der waals equation must be used to describe re...
Questions in other subjects:

Geography, 18.03.2021 15:20

Biology, 18.03.2021 15:20

Mathematics, 18.03.2021 15:20

History, 18.03.2021 15:20


Business, 18.03.2021 15:20





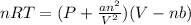 (1)
(1)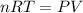 (2)
(2)



