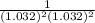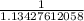Below regarding an electrochemical cell in an automotive lead-acid battery. the cell's anode is made of lead and the cathode is made of lead(iv) oxide. both are submerged in 4.30 m sulfuric acid. the half-reactions are: pbo_2(s) + 3h^+ (aq) + hso_4^- (aq) + 2e^- rightarrow pbso_4(s) + 2h_2o(l) e degree = 1.685 v pbso_4(s) + h^+(aq) + 2e^- rightarrow pb(s) + hso_4^- (aq) e degree = -0.356 v (a) calculate the value of e degree. (b) determine the initial value of e_cell. assume that the first ionization of h_2so_4 is complete and that [h^+] almostequalto [hso_4^-]. (c) find e_cell when the h^+ concentration has dropped by 76.00%. again, assume [h^+] almostequalto [hso_4^-].

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, pinkycupcakes3oxbqhx
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2


Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
Do you know the correct answer?
Below regarding an electrochemical cell in an automotive lead-acid battery. the cell's anode is made...
Questions in other subjects:


Mathematics, 27.07.2019 14:50





Geography, 27.07.2019 14:50


Mathematics, 27.07.2019 14:50


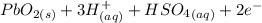 →
→ 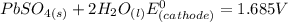
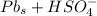 →
→ 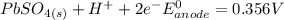
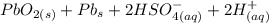 →
→ 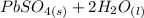
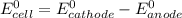
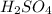 →
→ 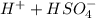
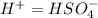 such that (;)
such that (;) 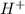 & b=
& b= 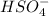
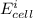 =
= 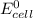 -
- 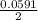 × log
× log 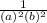
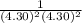
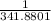
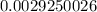
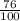
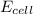 = 2.041V -
= 2.041V -