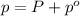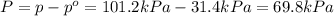Chemistry, 04.12.2019 04:31, alannaamarriee
Last week you reacted magnesium with a hydrochloric acid aqueous solution and hydrogen gas was produced. let's say that you collected the gas given off by the reaction and measured it's pressure as 101.2 kpa. if the vapor pressure of water is 31.4 kpa at this temperature, then what is the pressure of the hydrogen gas?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, dwighthibbert56
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
Do you know the correct answer?
Last week you reacted magnesium with a hydrochloric acid aqueous solution and hydrogen gas was produ...
Questions in other subjects:


Engineering, 14.10.2019 03:30

Mathematics, 14.10.2019 03:30


Physics, 14.10.2019 03:30




Geography, 14.10.2019 03:30


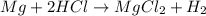
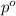 = 31.4 kilo Pascals
= 31.4 kilo Pascals