
The synthesis of methanol from carbon monoxide and hydrogen gas is described by the following chemical equation:
co(g)+2h2(g)⇌ch3oh(g)
the equilibrium constant for this reaction at 25 ∘c is kc=2.3×104. in this tutorial, you will use the equilibrium-constant expression to find the concentration of methanol at equilibrium, given the concentration of the reactants.
determine the expression for the equilibrium constant, kc, for the reaction by identifying which terms will be in the numerator and denominator:
the equilibrium-constant expression is a mathematical equation that can be rearranged to solve for any of the variables in it. rearrange the equilibrium-constant expression to solve for [ch3oh].
[ch3oh]=[ch3oh]=
1kc[co][h2]2
kc[co][h2]2
kc[co][h2]2
[co][h2]2kc

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, anarosa331hotmailcom
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 21.06.2019 22:20, aidengalvin20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 00:00, nyasiasaunders1234
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2
Do you know the correct answer?
The synthesis of methanol from carbon monoxide and hydrogen gas is described by the following chemic...
Questions in other subjects:

English, 04.12.2020 04:10



English, 04.12.2020 04:10


Mathematics, 04.12.2020 04:10



Spanish, 04.12.2020 04:10


![[CH_3OH]=K_c\times [CO]\times [H_2]^2](/tpl/images/0402/1764/fba08.png)
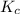
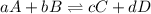
![K_{c}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0402/1764/b6f47.png)
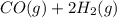 ⇌
⇌ 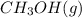
![K_{c}=\frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0402/1764/da8dd.png)





