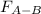Chemistry, 04.12.2019 03:31, christophercordero15
Two nonpolar organic liquids, hexane (c6h14) and heptane (c7h16), are mixed. do you expect δhsoln to be a large positive number, a large negative number, or close to zero? fill in the following sentence: δhsoln is determined by the relative magnitudes of the "old" solute-solute (δhsolute) and solvent-solvent (δhsolvent) interactions and the "new" solute-solvent (δhmix) interactions. in this process, the energy of mixing hexane and heptane (δhmix) is the energy of separating them individually (δhsolute + δhsolvent), so δhsoln is expected to be .

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1
Do you know the correct answer?
Two nonpolar organic liquids, hexane (c6h14) and heptane (c7h16), are mixed. do you expect δhsoln to...
Questions in other subjects:



Biology, 10.12.2019 05:31


Mathematics, 10.12.2019 05:31


Physics, 10.12.2019 05:31

Mathematics, 10.12.2019 05:31

Business, 10.12.2019 05:31

History, 10.12.2019 05:31


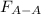 =
= 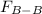 =
=