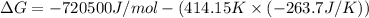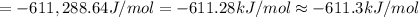Chemistry, 04.12.2019 03:31, ashcookie27
The value of delta g at 141.0 degrees celsius for the formation of phosphorous trichloride from its constituent elements,
p2(g) + 3cl2(g) > 2pcl3(g)
is kj/mol. at 25.0 degrees celsius for this reaction, delta h is -720.5 kj/mol, delta g is -642.9 kj/mol, and delta s is -263.7 j/k.
a.) -829.7
b.) 1.08 x 10^5
c.) 3.65 x 10^4
d.) -683.3
e.) -611.3

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 23.06.2019 07:00, ultimatesaiyan
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1
Do you know the correct answer?
The value of delta g at 141.0 degrees celsius for the formation of phosphorous trichloride from its...
Questions in other subjects:

Chemistry, 17.12.2020 22:50

Biology, 17.12.2020 22:50


Mathematics, 17.12.2020 22:50

Mathematics, 17.12.2020 22:50


Mathematics, 17.12.2020 22:50