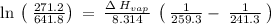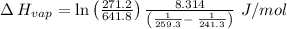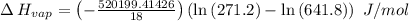Chemistry, 04.12.2019 03:31, gracynamos
The vapor pressure of the liquid so2 is measure at different temperatures. the following vapor pressure data are obtained. temp (k) pressure mmhg241.3 271.2259.3 641.8calculate the enthalpy of vaporization(delta h vap) in kj/mol for this liquid.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
Do you know the correct answer?
The vapor pressure of the liquid so2 is measure at different temperatures. the following vapor press...
Questions in other subjects:


Mathematics, 20.11.2021 03:50

Mathematics, 20.11.2021 03:50

Mathematics, 20.11.2021 03:50

Computers and Technology, 20.11.2021 03:50



Mathematics, 20.11.2021 03:50



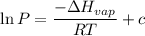
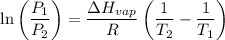
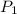 = 271.2 mmHg
= 271.2 mmHg
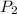 = 641.8 mmHg
= 641.8 mmHg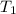 = 241.3 K
= 241.3 K 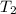 = 259.3 K
= 259.3 K