
Chemistry, 04.12.2019 02:31, dancer2814
Line spectra from all regions of the electromagnetic spectrum, including the paschen series of infrared lines for hydrogen, are used by astronomers to identify elements present in the atmospheres of stars. calculate the wavelength of the photon emitted when the hydrogen atom undergoes a transition from n = 5 to n = 3. (r = 2.179 x 10-18 j r = 1.096776 x 10^7 m-1) a. 205.1 nm b. 384.6 nm c. 683.8 nm d. 1282 nm e. > 1500 nm

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, WhiteWinterRose
What is the chemical formula of the following compound
Answers: 3

Chemistry, 21.06.2019 22:30, connienash95
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
Do you know the correct answer?
Line spectra from all regions of the electromagnetic spectrum, including the paschen series of infra...
Questions in other subjects:



Mathematics, 12.06.2021 01:40



Mathematics, 12.06.2021 01:40


Arts, 12.06.2021 01:40


Mathematics, 12.06.2021 01:40


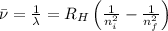
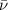 = Wave number
= Wave number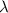 = Wavelength of radiation
= Wavelength of radiation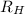 = Rydberg's Constant
= Rydberg's Constant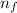 = Higher energy level
= Higher energy level 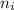 = Lower energy level
= Lower energy level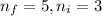
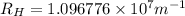
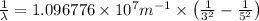
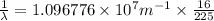
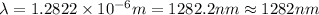
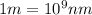 )
)



