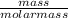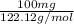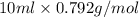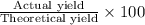Chemistry, 04.12.2019 00:31, Aydenj9613
Assume that you react 100 mg of benzoic acid with 10 ml of methanol and 10 microliters of sulfuric acid to produce methyl benzoate. write a balance chemical equation for this reaction. determine the limiting reagent and calculate a theoretical yield of both the ester and water. if you isolate 75 mg of methyl benzoate, what is the actual yield of the reaction?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 09:20, nyceastcoast
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1
Do you know the correct answer?
Assume that you react 100 mg of benzoic acid with 10 ml of methanol and 10 microliters of sulfuric a...
Questions in other subjects:


Mathematics, 18.11.2020 21:50

English, 18.11.2020 21:50

History, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50

History, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50


English, 18.11.2020 21:50


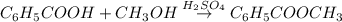
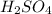 is very small so, that is catalytic amount of
is very small so, that is catalytic amount of