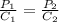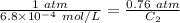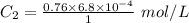Chemistry, 04.12.2019 00:31, fireman59937
The solubility of nitrogen gas at 25 ◦c and 1 atm is 6.8×10−4 mol/l. if the partial pressure of nitrogen gas in air is 0.76 atm, what is the concentration (molarity) of dissolved nitrogen?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Do you know the correct answer?
The solubility of nitrogen gas at 25 ◦c and 1 atm is 6.8×10−4 mol/l. if the partial pressure of nitr...
Questions in other subjects:







History, 29.06.2019 16:50



History, 29.06.2019 16:50