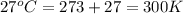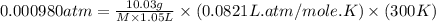Chemistry, 04.12.2019 00:31, zuleidysnegron
An aqueous solution of 10.03 g of catalase, an enzyme found in the liver, has a volume of 1.05 l at 27°c. the solution's osmotic pressure at 27°c is found to be 0.745 torr. calculate the molar mass of g/mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, zaehairston78531
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1


Chemistry, 21.06.2019 23:00, LarryJoeseph
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1
Do you know the correct answer?
An aqueous solution of 10.03 g of catalase, an enzyme found in the liver, has a volume of 1.05 l at...
Questions in other subjects:


Mathematics, 11.02.2020 06:02

Mathematics, 11.02.2020 06:02


Mathematics, 11.02.2020 06:02

Mathematics, 11.02.2020 06:02

Mathematics, 11.02.2020 06:02

Mathematics, 11.02.2020 06:02


History, 11.02.2020 06:02


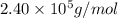
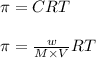
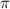 = osmotic pressure = 0.745 torr = 0.000980 atm (1 atm = 760 torr)
= osmotic pressure = 0.745 torr = 0.000980 atm (1 atm = 760 torr)