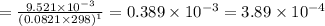Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 23.06.2019 12:00, jluvit6135
In a reduction half-reaction, which amount is shown
Answers: 3
Do you know the correct answer?
Consider the following equilibrium.2 nobr(g)< => 2 no(g) + br2(g)if nitrosyl bromide, nobr, i...
Questions in other subjects:



Mathematics, 08.12.2020 01:00

Health, 08.12.2020 01:00

English, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00



Social Studies, 08.12.2020 01:00


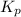 of the reaction is
of the reaction is 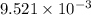 .
.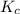 of the reaction is
of the reaction is 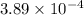 .
.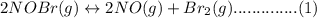
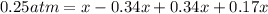
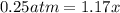
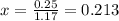
![[]P_{NOBr}]](/tpl/images/0401/8095/a6c6d.png) is 0.14 atm.
is 0.14 atm.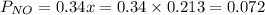
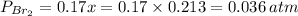
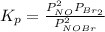
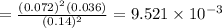
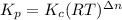
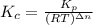
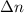 = number of moles of reactants - Number of moles of products
= number of moles of reactants - Number of moles of products