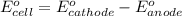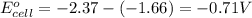Consider a galvanic cell based on the reaction al^3+_(aq) + mg_(s) rightarrow al_(s) + mg^2+ _(aq) the half-reactions are al^3+ + 3 e^- rightarrow al e degree = - 1.66 v mg^2+ + 2 e^- rightarrow mg e degree = - 2.37 v give the balanced cell reaction and calculate e degree for the cell.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 23:00, Mynameismath
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
Do you know the correct answer?
Consider a galvanic cell based on the reaction al^3+_(aq) + mg_(s) rightarrow al_(s) + mg^2+ _(aq) t...
Questions in other subjects:

Physics, 06.11.2020 02:30

Mathematics, 06.11.2020 02:30


English, 06.11.2020 02:30

Arts, 06.11.2020 02:30


Mathematics, 06.11.2020 02:30


Mathematics, 06.11.2020 02:30

Biology, 06.11.2020 02:30


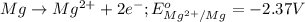 ( × 3)
( × 3)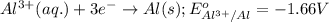 ( × 2)
( × 2)
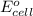 of the reaction, we use the equation:
of the reaction, we use the equation: