
Chemistry, 03.12.2019 22:31, allieballey0727
Given that h 2 ( g ) + f 2 ( g ) ⟶ 2 hf ( g ) δ h ∘ rxn = − 546.6 kj 2 h 2 ( g ) + o 2 ( g ) ⟶ 2 h 2 o ( l ) δ h ∘ rxn = − 571.6 kj calculate the value of δ h ∘ rxn for 2 f 2 ( g ) + 2 h 2 o ( l ) ⟶ 4 hf ( g ) + o 2 ( g )

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, destineysarah
98 points you will be galileo perform the experiment to determine if objects with different mass fall at the same, or different, rates in the air and in a vacuum. before you conduct your experiment, you need to form a hypothesis. a hypothesis is a prediction of what you think will happen in the experiment. the hypothesis is a statement that describes “if” a certain set of circumstances are present “then” there will be a specific result that will occur. record your hypothesis here: record the results from step one of the experiment (dropping the objects in the air): first trial: second trial: third trial: record the results from step two of the experiment (dropping the objects in a vacuum): first trial: second trial: third trial: did the experiment support your hypothesis? using the data from your experiment, describe why you believe your hypothesis was either proven or disproven. what forces were acting on the objects dropped in the air? what force was acting on the objects dropped in the vacuum? part two: comparing forces choose two forces and compare and contrast these forces. you must provide two ways that they are alike and two ways that they are different. you may make a list, write in paragraph form, or make a chart. choose two forces and compare and contrast these forces. these must be different forces than used in the prior question. provide two ways that they are similar and two ways that they are different. you may make a list, write it out, or make a chart.
Answers: 3

Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 04:20, tyrickdavis1
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1

Chemistry, 23.06.2019 11:30, junahsisney
Bridget is in science class. her teacher gives her two unknown substances and asks her to determine their relative ph. she places a piece of red litmus paper into both substances. the litmus paper turns purple when she places it into substance i. the litmus paper turns blue when she places it into substance ii. a. substance i is a neutral substance and substance ii is an acid. b. substance i is a neutral substance and substance ii is a base. c. substance i is an acid and substance ii is a base. d. substance i is a base and substance ii is a neutral substance.
Answers: 1
Do you know the correct answer?
Given that h 2 ( g ) + f 2 ( g ) ⟶ 2 hf ( g ) δ h ∘ rxn = − 546.6 kj 2 h 2 ( g ) + o 2 ( g ) ⟶ 2 h 2...
Questions in other subjects:

Mathematics, 21.10.2020 01:01



History, 21.10.2020 01:01


Biology, 21.10.2020 01:01




Health, 21.10.2020 01:01


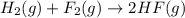
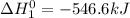 (1)
(1)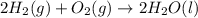
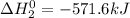 (2)
(2)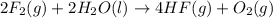
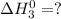 (3)
(3)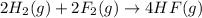
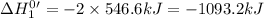 (1')
(1')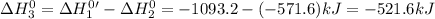 .
.



