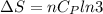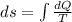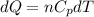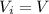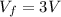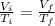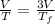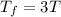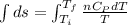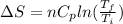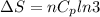Chemistry, 03.12.2019 21:31, squidmeat12
Asample consisting of n mol of an ideal gas undergoes a reversible isobaric expansion from volume vi to volume 3vi. find the change in entropy of the gas by calculating, ∫dq / t, where dq = ncpdt. (use the following as necessary: cp and n.)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:00, erickamurillo9929
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2


Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1
Do you know the correct answer?
Asample consisting of n mol of an ideal gas undergoes a reversible isobaric expansion from volume vi...
Questions in other subjects:


Geography, 22.10.2020 22:01


Mathematics, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01

History, 22.10.2020 22:01