
Chemistry, 03.12.2019 21:31, courtnicollee
Fe(ii) can be precipitated from a slightly basic (aq) solution by bubbling oxygen through the solution, which converts fe(ii) to insoluble fe(iii): 4fe(oh)+(aq)+ 4oh-(aq)+o2(g)+2h2o(l)=4fe(oh)3(s)h ow many grams of o2 are consumed to precipitate all of the iron in 85ml of 0.075 m fe(ii).

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:02, dondre54
Use the image below to answer the following questions there are three types of heat transfer occurring in the picture below. write a sentence about where each is occurring. (total three complete sentences in your own words.) on a very cold day, if you place your hand towards the bottom of a window, you can feel the convection of cold air as the cold, dense air sinks. try it out. did you feel the convection? it's a marshmallow on a metal stick over fire
Answers: 3

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 03:30, HalpMahOnMahH0meW0rk
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
Do you know the correct answer?
Fe(ii) can be precipitated from a slightly basic (aq) solution by bubbling oxygen through the soluti...
Questions in other subjects:

Biology, 19.12.2019 12:31


Mathematics, 19.12.2019 12:31

Social Studies, 19.12.2019 12:31





Social Studies, 19.12.2019 12:31


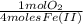 = 1,6x10⁻³ moles of O₂(g)
= 1,6x10⁻³ moles of O₂(g)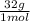 = 0,051g of O₂ are consumed
= 0,051g of O₂ are consumed



