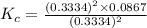Chemistry, 03.12.2019 21:31, maxi12312345
At a certain temperature, 0.760 mol so3 is placed in a 1.50 l container. 2so3(g) = 2so2(g) + o2(g)at equilibrium, 0.130 mol o2 is present. calculate kc.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:40, ellemarshall13
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
Do you know the correct answer?
At a certain temperature, 0.760 mol so3 is placed in a 1.50 l container. 2so3(g) = 2so2(g) + o2(g)at...
Questions in other subjects:


Mathematics, 02.02.2021 07:40

English, 02.02.2021 07:40

Social Studies, 02.02.2021 07:50

Chemistry, 02.02.2021 07:50

SAT, 02.02.2021 07:50

Mathematics, 02.02.2021 07:50

Mathematics, 02.02.2021 07:50



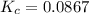




![K_c=\frac {[SO_2]^2[O_2]}{[SO_3]^2}](/tpl/images/0401/5810/3722c.png)