

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
Do you know the correct answer?
Consider the fructose-1,6-bisphosphatase reaction. calculate the free energy change if the ratio of...
Questions in other subjects:


History, 18.12.2020 17:40

Mathematics, 18.12.2020 17:40

History, 18.12.2020 17:40


Business, 18.12.2020 17:40






 = free energy of the reaction
= free energy of the reaction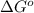 = standard Gibbs free energy = -16.7 kJ/mol = -16700 J/mol (Conversion factor: 1kJ = 1000J)
= standard Gibbs free energy = -16.7 kJ/mol = -16700 J/mol (Conversion factor: 1kJ = 1000J)
![37^oC=[273+37]K=310K](/tpl/images/0401/5815/c6b28.png)
 = Ratio of concentration of products and reactants = 21.3
= Ratio of concentration of products and reactants = 21.3





