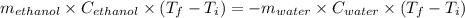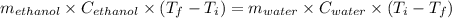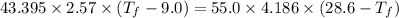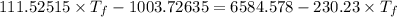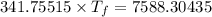Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 06:30, lainnn974
(04.01 lc) which of the following is true about science? (5 points) select one: a. it is not influenced by social conditions. b. it is not determined by external local factors. c. political conditions are unable to influence it. d. economic concerns may prevent it from solving problems.
Answers: 1


Chemistry, 23.06.2019 14:40, sophiamoser
Uuestons niuthe no. of millimoles of hcl required to neutralize 10 ml of 0.2 m na2co3 is(a) 2.0 m mole(b) 4.0 m mole(c) 0.2 m mole(d) 0.4 m mole
Answers: 1
Do you know the correct answer?
If 55.0 ml of ethanol (density=0.789g/ml)) initially at 9.0 ∘c is mixed with 55.0 ml of water (densi...
Questions in other subjects:

Chemistry, 07.05.2021 21:10


Mathematics, 07.05.2021 21:10

Mathematics, 07.05.2021 21:10

Mathematics, 07.05.2021 21:10




Mathematics, 07.05.2021 21:10